Heat capacity ratio of heat absorbed by a material to the temperature change. In the previous article we discussed the specific heat capacity of substances.

Pin By Redacted On Chemistry Education Chemistry Education What Is Science Ap Chem
In a coffee cup calorimeter the reaction takes place in the water while in a bomb calorimeter the reaction takes place in a sealed metal container which.
. Thermochemistry determine the heat exchanged at constant pressure q m c T. Back to top Quick links to sections of this page. This value and the measured increase in temperature of the calorimeter can be used to determine C bomb.
Differential scanning calorimeters isothermal micro calorimeters titration calorimeters and accelerated rate calorimeters are among the most common types. A simple calorimeter just consists of a thermometer attached to a. Both solutions were originally at 261C.
Heat is measured by the principle of calorimetry. The definition of the calorie is based on the specific heat of water defined. Where ΔU is change in internal energy ΔT is change in temperature and C V is the heat capacity at constant volume.
A calorimeter is a device designed to measure heat of reaction or physical changes and heat capacity. Heat capacity is a measurable physical quantity equal to the ratio of the heat added to an object to the resulting. Heat transferred to calorimeter Q mcΔT.
Place one litre 1 kg of water in the calorimeter. It is usually expressed as calories per degree in terms of the actual amount of material being considered most commonly a mole the molecular weight in grams. The heat capacity in calories per gram is called specific heat.
C is the specific heat of a material JgK. After waiting for the system to equilibrate the final temperature reached is 283 C. A bomb calorimeter is used to measure heat flows for gases and high-temperature reactions.
The device can be sophisticated and expensive or simple and cheap. An alternative truly measuring at operational conditions lies in using an adiabatic flow calorimeter and thus evaluating the enthalpy balance of a small quantity of the HTF in a bypass of the system 5. Calorimeters have been designed in great variety.
A calorimeter is a device designed to measure heat of reaction or physical changes and heat capacity. Energy is defined as the capacity to do work. Q is the heat absorbed or released by a material J.
Heat capacity of calorimeter C183JC Increase in temperature of calorimeter question_answer Q. M is the mass of a material g. When it is connected to the system by a path for heat transfer changes in it measure heat transfer.
Heat Capacity of Calorimeter 500 mL of water at 405 C is added to a calorimeter containing 500 mL of water at 174 C. Find the heat transferred to the calorimeter if the heat capacity of the calorimeter is 893 kJK. Specific heat capacity is the most useful quantity available from DSC because it is directly related to sample properties and.
A 435-g sample of copper at 999 C is dropped into a beaker containing 156 g of water at 187 C. A calorimeter consists of two vessels outer vessel and an inner vessel. The formula for power is.
Assuming that all the solutions have a density of 10 gcm3 and a specific heat capacity of 418 JCg calculate the enthalpy change for the neutralization of HCl by NaOH. The temperature of the calorimeter rises from 250 to 310 K. Science Chemistry QA Library In a coffee-cup calorimeter 1200 mL of 10 M NaOH and 1200 mL of 10 M HCl are mixed.
Heat capacity c 893 kJk. One type in widespread use called a bomb calorimeter basically consists of an enclosure in which the reaction takes place surrounded by a liquid such as water that absorbs. A calorimeter is an object used for calorimetry or the process of measuring the heat of chemical reactions or physical changes as well as heat capacityDifferential scanning calorimeters isothermal micro calorimeters titration calorimeters and accelerated rate calorimeters are among the most common types.
The SI unit of energy is Joules J. Clamp the thermometer into the smaller hole with the stirrer. The specific heat capacity of a substance usually denoted by or s is the heat capacity of a sample of the substance divided by the mass of the sample.
Table of Specific Heat Capacities. A 196 g sample of titanium was burned in a bomb calorimeter. Calculating the limiting reactant the change in enthalpy of the reaction H rxn can be determined since the reaction was conducted under conditions of constant pressure H rxn q rxn moles of limiting reactant.
HClaq NaOHaq -- NaClaq H 2 Ol Energy. The heat capacity at constant volume is assumed to be independent of temperature. Rise in temp ΔT 310250 K 60 K.
Where represents the amount of heat needed to uniformly raise the temperature of the sample by a small increment. Answer- Firstly using the given heat mass and temperature change specific heat capacity is Q. T 2 T 1 is the temperature difference before and after heating or cooling K.
Mass of octane m 1750g. Such measurements can be made easily with this. The method of mixture is used almost universally by scientists as a quick easy and semi-accurate specific heat test for a solid sample but what makes this method extra special is the fact that its so simple that high school students around the world perform it as a hands-on example of how the specific heat capacities of materials are a part of the world around us.
Sp_heat of water 4184 JgC Δt hot 283 C - 405 C. Say in a calorimeter a fixed amount of fuel is burned. A calorimeter is a body in the surroundings of the system with its own temperature and internal energy.
The specific heat also called specific heat capacity is the measure of the heat energy that a substance in a unit quality absorbs or releases when the. Heat loss by the fuel is equal to the heat gained by the water. Benzoic acid C 6 H 5 CO 2 H comb 2638 kJg.
After the reaction the final temperature is 328C. The vessel is filled with water and the fuel is burned leading to the heating of the water. List of thermal conductivities Note that the especially high molar values as for paraffin gasoline water and ammonia result from calculating specific heats in terms of moles of molecules.
To measure the heat capacity of the calorimeter we first burn a carefully weighed mass of a standard compound whose enthalpy of combustion is accurately known. The device can be sophisticated and expensive or simple and cheap. The energy stored in an object due to its position and height is known as potential energy and is given by the formula.
Power is defined as the rate at which work is done. A bomb calorimeter works in the same manner as a coffee cup calorimeter with one big difference. The bomb calorimeter has a heat.
A calorimeter is an object used for calorimetry or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity. Specific heat capacity of water c g 4184 J C-1 g-1 from a data sheet mass water 200 g from the experiment. Calorimeter device for measuring the heat developed during a mechanical electrical or chemical reaction and for calculating the heat capacity of materials.
Like the heat capacity of an object the specific heat capacity of a substance may vary. Calculate the heat capacity of the calorimeter. Place the immersion heater into the central hole at the top of the calorimeter.
A calorimeter consists of two vessels outer vessel and an inner vessel. If specific heat is expressed per mole of atoms for these substances none of the constant-volume values exceed to any large extent the theoretical. An alternative method for determining heat of combustion enthalpy of combustion using a bomb calorimeter is outlined in the calorimetry tutorial.

Constant Volume Calorimetry For More Precise Work Than The Coffee Cup Calorimeter The Heat Capacity Of The Coffee Cups Chemistry Education Chemical Reactions

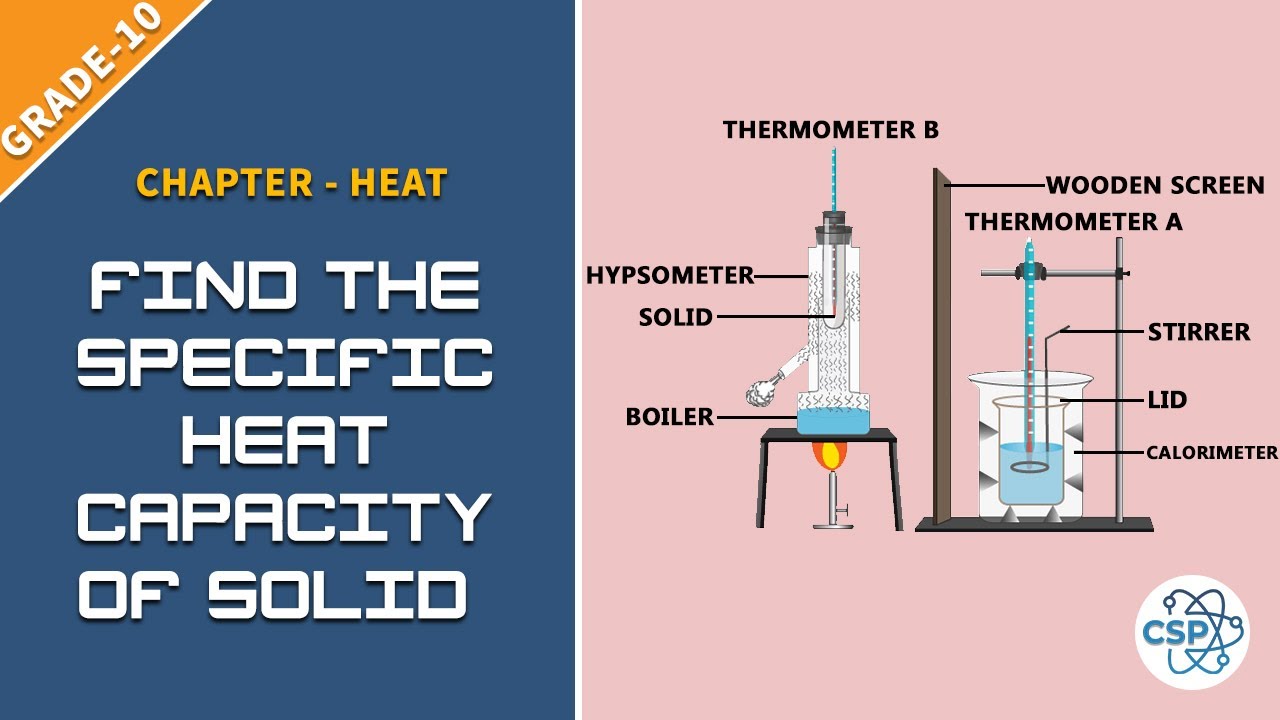
To Find The Specific Heat Capacity Of Solid By Using Method Of Mixtures See Class 10 Physical Properties Heat Science Experiments

50 Calorimetry Worksheet Answer Key Chessmuseum Template Library Worksheets Capacity Worksheets Letter Reversals

Basic Calorimeter For Measuring Heat Capacity Is A Foam Coffee Cup Calorimeter Heatcapacity Coffee Cups Heat Heat Transfer
0 Comments